No products in the basket.
Transitioning HBV cure programs into multiple late-stage combination studies with interim results throughout 2024 and 2025 informing Company’s registrational strategy
Integrating R&D, manufacturing and commercial upsides by acquiring full intellectual property rights of BRII-179 and expanding its manufacturing footprint
Prioritizing company resources with a robust cash balance of US$376 million supporting operations until 2027
Company to host a conference call (English session) on March 25 at 8:30 AM ET / 8:30 PM HKT
DURHAM, N.C. and BEIJING, March 22, 2024 /PRNewswire/ — Brii Biosciences Limited (“Brii Bio” or the “Company”, stock code: 2137.HK), a biotechnology company developing therapies to improve patient health and choice across diseases with high unmet need, today provided a corporate update and reported its financial results for the year ended December 31, 2023.
Throughout 2023, the Company delivered significant milestones in its leading HBV program, driving the development of novel combination treatment regimens for an HBV functional cure while also expanding its portfolio. Following formative data readouts from ongoing trials with multiple HBV candidates and the acquisition of full intellectual property rights for BRII-179 and its technology transfer, Brii Bio is poised to launch multiple combination studies in 2024.
“2023 was a pivotal year for Brii Bio, with key insights received from multiple Phase 2 combination studies,” stated Zhi Hong, Ph.D., Chairman and Chief Executive Officer of Brii Bio. “Through vigorous clinical investigations over the past 5 years, we have a deep understanding of what is required to maximally reduce and sustain HBsAg loss. With BRII-179 we have a strategy to assess and enhance HBV patients’ intrinsic immunity, targeting therapies to those most likely to respond, while sparing others from poorly tolerated regimens. These are important breakthroughs informing our late-stage clinical combination trials.”
Full Year 2023 and Recent Corporate Updates
- In February 2024, Brii Bio finalized agreements with VBI Vaccines, Inc. (“VBI”, NASDAQ: VBIV) to acquire full intellectual property rights for BRII-179 and manufacturing technology, to expand its future global manufacturing and supply capabilities. This move also eliminates future payments to VBI related to BRII-179 and PreHevbriTM.
- BRII-179 received Breakthrough Therapy Designation (BTD) from the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) in November 2023, expediting innovative treatments for HBV patients.
- Brii Bio secured exclusive development and commercialization rights for PreHevbriTM in Greater China and Asia Pacific (excluding Japan) in July 2023 and has submitted two pre-INDs to the CDE for PreHevbriTM‘s registration plan in China. A Market Authorization Application has also been filed in Hong Kong.
- Brii Bio has prioritized resources to focus on the clinical and commercial development of its advanced HBV portfolio. The Company is actively seeking partnership opportunities for further development of its CNS, HIV, and MDR/XDR global assets.
- Brii Bio has appointed Dr. Brian A. Johns as Chief Scientific Officer (CSO) to oversee Brii Bio’s discovery programs and shape the Company’s future pipeline strategy. Our new organizational strategy is underpinned by highly experienced leaders in infectious diseases who are committed to clear deliverables across the Company’s pipeline.
Full Year 2023 Core Clinical Pipeline Highlights and Upcoming Milestones
Hepatitis B Virus (HBV) Program
Key data were generated in 2023 confirming that BRII-179, our proprietary therapeutic vaccine, can trigger a potent and specific immune response in chronic Hepatitis B (CHB) patients and linking that response to improved clinical outcomes. Brii Bio, with our strategic partners, is conducting a series of confirmatory combination studies in 2024, to establish BRII-179 as a core component of future curative regimens in Hepatitis B. In furthering our commitment to BRII-179, Brii Bio negotiated full IP and manufacturing rights with VBI. We are working closely with our partners to initiate late-stage clinical studies to definitively address the contribution of BRII-179 and BRII-835 (elebsiran) towards achieving a higher functional cure rate of HBV infection. More importantly, BRII-179 holds the potential to identify immune-responsive CHB patients with a higher chance of achieving a functional cure.
Key Data and Development Plans:
- BRII-179 Related Studies and Plans
- Brii Bio presented two late-breaking posters on BRII-179 at the American Association for the Study of Liver Diseases (AASLD) The Liver Meeting® in November 2023, showing an important connection between HBsAg loss and anti-HBs antibody response. These data enable a clear direction in further improving the functional cure rate and identifying patients likely to respond to curative treatments.
– BRII-179 induced functional immune responses that improve the rate and duration of HBsAg loss in CHB patients who receive PEG-IFNα treatment, thereby increasing the CHB functional cure rate.
– Translational research data from Brii Bio’s Phase 1b/2a studies on BRII-179 and BRII-179 in combination with BRII-835 (elebsiran) suggests that BRII-179 may offer a unique opportunity to identify CHB patients who are able to elicit the necessary HBV-specific immune responses hence achieving higher functional cure rate in the selected patients while sparing others from unnecessary treatments.
- Brii Bio plans to initiate additional combination studies in the second half of 2024 to confirm the ability of BRII-179 to enhance HBV functional cure rates in combination with other modalities.
- Brii Bio will present data on patients meeting NRTI discontinuation criteria from its ongoing Phase 2 study of BRII-179 in combination with PEG-IFNα in CHB patients as an oral late-breaking presentation at the 33rd Conference of Asian Pacific Association for the Study of the Liver (APASL 2024), being held from March 27 – 31, 2024 in Kyoto, Japan.
- BRII-835 (Elebsiran) & BRII-877 (Tobevibart) Related Studies and Plans
- Completed enrollment in the Phase 2 study of BRII-835 (elebsiran) in combination with PEG-IFNα in the APAC region including mainland China. The primary objectives of this study are to gain deeper insights into the contribution of BRII-835 (elebsiran) towards improving HBV cure rates relative to PEG-IFNα alone and to explore the role of BRII-179 in enriching patients to achieve better curative outcomes. Early topline result is expected in the fourth quarter of 2024.
- Brii Bio’s development partner, Vir Biotechnology, Inc. (“Vir”, Nasdaq: VIR), presented new MARCH Part B data at the AASLD The Liver Meeting in November 2023. The data demonstrated an approximately three-fold higher response rate when adding BRII-877 (tobevibart) to a regimen of BRII-835 (elebsiran) with or without PEG-IFNα after 24 weeks of treatment: 15.0% for BRII-877 (tobevibart) + BRII-835 (elebsiran) + PEG-IFNα and 14.3% for BRII-877 (tobevibart) + BRII-835 (elebsiran). The data from cohorts undergoing 48 weeks of treatment will be available in the fourth quarter of 2024.
- In a late-breaker presentation at the AASLD The Liver Meeting in November 2023 with an update at the 42nd Annual J.P. Morgan Healthcare Conference in January 2024, Vir shared initial SOLSTICE data from a small subset of chronic hepatitis D (CHD) participants. After 12 weeks of combination treatment with BRII-877 (tobevibart) and BRII-835 (elebsiran), 5 of 6 participants achieved undetectable HDV RNA and 6 out of 6 were below the lower limit of quantitation. Additional data will be presented in the second quarter of 2024 and complete 24-week treatment data are expected in the fourth quarter of 2024.
- A Phase 1 study of BRII-877 (tobevibart) is ongoing in China. Human pharmacokinetics (PK) in mainland Chinese subjects will be compared to subjects from the APAC region and Europe.
Additional Clinical and Pre-Clinical Development Updates
Based on the Company’s strategy to focus on its advanced HBV programs, Brii Bio is pursuing partnership opportunities for the continued development of these programs.
Multidrug- and Extensively Drug-Resistant (MDR/XDR) Gram-Negative Bacteria Infections Program
- BRII-693
- Brii Bio gained exclusive global rights in June 2023 to develop and commercialize BRII-693, a novel polymyxin for the treatment of serious gram-negative infections. The Company is seeking strategic funding partners to expedite development of BRII-693, a novel antibiotic intended to help tackle the growing worldwide threat of antimicrobial resistance.
- In April 2023, Brii Bio submitted a pre-IND to the NMPA of China for the development of BRII-693, with plans to initiate one Phase 1 PK bridging study in China. Additional clinical PK studies to enable the initiation of a Phase 3 trial are in planning. These studies are crucial to support global development efforts. A large, global Phase 3 registrational trial in hospital-acquired bacterial pneumonia (HABP)/ventilator-associated bacterial pneumonia (VABP), is expected to begin in 2025.
Human Immunodeficiency Virus (HIV) Infection Program
- BRII-753 is an internally discovered new chemical entity currently in the preclinical stage of development. BRII-753 is being developed as a long-acting subcutaneous injection with the potential for dosing once monthly, once quarterly, or twice yearly and can be used in combination therapy for HIV treatment and as monotherapy for pre-exposure prophylaxis (PrEP).
- BRII-732 has completed Phase 1 studies with the potential for development as part of an oral, once-weekly, long-acting combination treatment option for HIV patients.
Non-Tuberculous Mycobacterial (NTM) Lung Disease Program
- BRII-658 (Epetraborole): In February 2024, Brii Bio’s partner, AN2 Therapeutics, Inc. (NASDAQ: ANTX) announced a voluntary pause of its Phase 3 enrollment in a Phase 2/3 clinical trial for epetraborole (BRII-658) for the treatment-refractory Mycobacterium avium complex (MAC) lung disease, pending further data review.
Postpartum Depression (PPD) and Major Depressive Disorder (MDD)/Other CNS Disorders
- BRII-296: In September 2023, the first patient was dosed in the Phase 2 study evaluating BRII-296, a long-acting injectable (LAI) therapy in development for PPD. The Company expects data readouts from the Phase 2 trial by the second quarter of 2024.
- BRII-297: Dosing has been completed in a Phase 1 clinical trial for BRII-297, a long-acting injectable (LAI) being developed to treat anxiety and depressive disorders. The study aims to evaluate the safety, tolerability, and PK of BRII-297 in healthy volunteers with data expected by the second half of 2024.
Full Year 2023 Financial Results
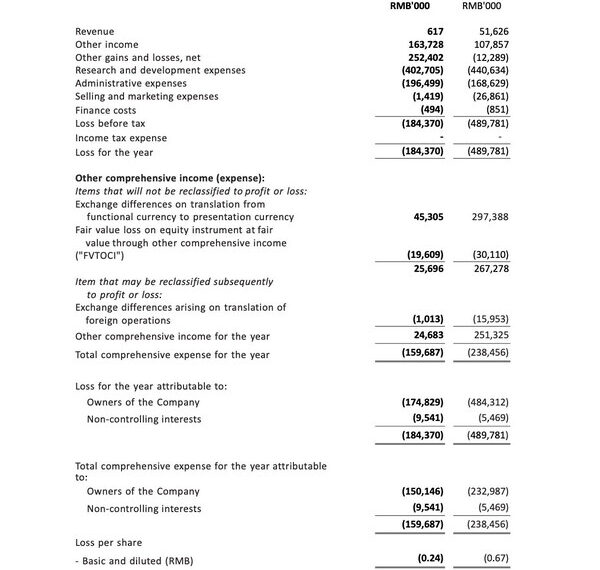
- Our bank deposits and cash and cash equivalents were RMB2,661.4 million as of December 31, 2023, representing a decrease of 337.9 million or 11.3%, compared with RMB2,999.3 million as of December 31, 2022. The decrease was primarily due to payout of daily operations and research and development activities.
- Other income was RMB163.7 million for the year ended December 31, 2023, representing an increase of RMB55.8 million or 51.7%, compared with RMB107.9 million for the year ended December 31, 2022. This was mainly due to the increased bank interest income of RMB70.8 million attributable to the rising interest rates on USD and HKD time deposits. The increase was partially offset by the decrease in income recognized from PRC government grants.
- Research and development expenses were RMB402.7 million for the year ended December 31, 2023, representing a decrease of RMB37.9 million or 8.6%, compared with RMB440.6 million for the year ended December 31, 2022. The decrease was primarily due to the decrease in discontinuation of COVID-19 program.
- Administrative expenses were RMB196.5 million for the year ended December 31, 2023, representing an increase of RMB27.9 million or 16.5%, compared with RMB168.6 million for the year ended December 31, 2022. The increase was primarily attributable to the increase in employee cost.
- Total comprehensive expense for the year ended December 31, 2023, was RMB159.7 million, representing a decrease of RMB78.8 million or 33.0 %, compared with RMB238.5 million for the year ended December 31, 2022. The decrease was primarily attributable to the increase in other gain and loss, which was partially offset by the decrease in gain arising from the exchange difference on translation from functional currency to presentation currency.
Conference Call Information
A live conference call (English session) will be hosted on March 25, 2024 at 8:30 AM U.S. Eastern Time (8:30 PM Hong Kong Time). All participants are required to register in advance of the call. For the registration link, please click here.
All participants shall use the link provided above to complete the online registration process in advance of the conference call. Upon registering, each participant will receive an email with important details for this call, including the call date, time and access link. This link is to be kept confidential and not shared with other participants. Additionally, a replay of the conference call will be available after the call and can be accessed by visiting the Company’s website at www.briibio.com under the Investor Relations section.
***
This press release contains references to third-party information. Such information is not deemed to be incorporated by reference in this press release. Brii Bio disclaims responsibility for such third-party information.
About Brii Bio
Brii Biosciences Limited (“Brii Bio“, stock code: 2137.HK) is a biotechnology company developing therapies to address major public health challenges where patients experience high unmet medical needs, limited choice and significant social stigmas. With a focus on infectious and central nervous system diseases, the Company is advancing a broad pipeline of unique therapeutic candidates with lead programs against hepatitis B viral infection (HBV), postpartum depression (PPD), and major depressive disorder (MDD). The Company is led by a visionary and experienced leadership team and has operations in key biotech hubs, including Raleigh-Durham, the San Francisco Bay Area, Beijing and Shanghai. For more information, visit www.briibio.com.
Forward Looking Statement
The information communicated in this press release contains certain statements that are or may be forward looking. These statements typically contain words such as “will,” “expects,” “believes,” “plans” and “anticipates,” and words of similar import. By their nature, forward looking statements involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. There may be additional material risks that are currently not considered to be material or of which the Company are unaware. These forward-looking statements are not a guarantee of future performance. Against the background of these uncertainties, readers should not rely on these forward-looking statements. The Company assumes no responsibility to update forward-looking statements or to adapt them to future events or developments.
Source : Brii Biosciences Provides Corporate Update and Reports Full-Year 2023 Financial Results
The information provided in this article was created by Cision PR Newswire, our news partner. The author's opinions and the content shared on this page are their own and may not necessarily represent the perspectives of Thailand Business News.
Discover more from Thailand Business News
Subscribe to get the latest posts sent to your email.
Please login to join discussion